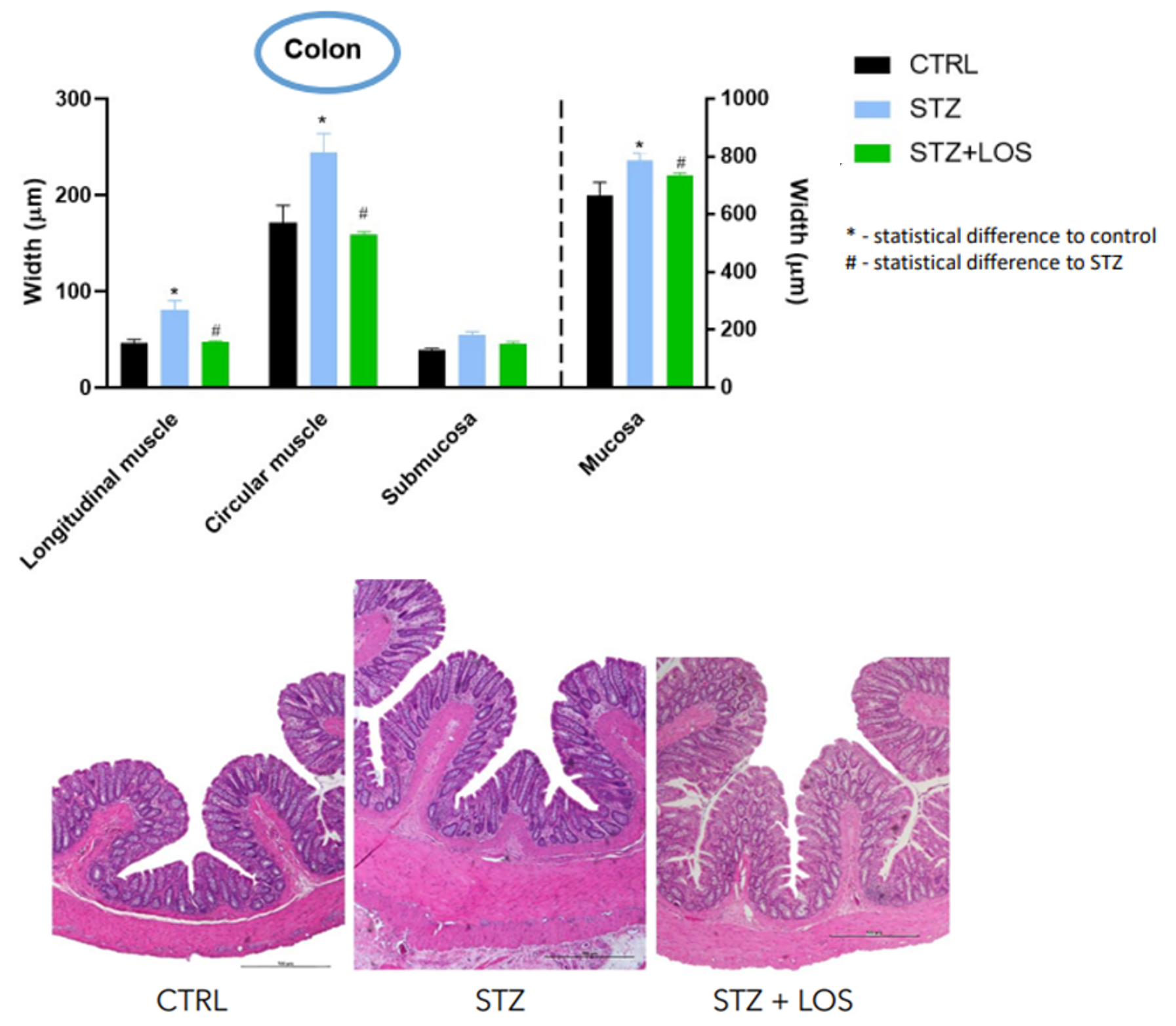
The presented invention is based on redirecting the use of ACEIs and ARAs to prevent and/or revert diabetic-induced GI complications, that are associated with morbidity, affecting up to 75% of patients.
Diabetes mellitus (DM) is a complex chronic progressive metabolic disorder, medically incurable, that can affect almost every organ system with a prevalence in humans of all age groups at alarming proportions worldwide. Gastrointestinal (GI) complications are very common and often result in symptoms like chronic constipation, diarrhea (occasionally associated with fecal incontinence), and are associated with worse outcomes in clinical conditions like colorectal cancer and inflammatory bowel disease.
Additionally, possible complications like megacolon, pseudo-obstruction, stercoral ulcer or perforation may occur. Despite the high prevalence of these GI complications, the knowledge and treatment options for these are considerably scarce.
As mentioned, there is no medication designated specifically to prevent or treat GI alterations associated with DM. The existing treatments primarily focuses on symptomatic management and are very limited. Therefore, this invention comprises a new area of therapeutic intervention, not previously described.
Fresh therapeutic application for ACEIs & ARAs, for both human and companion animals use.






